-40%
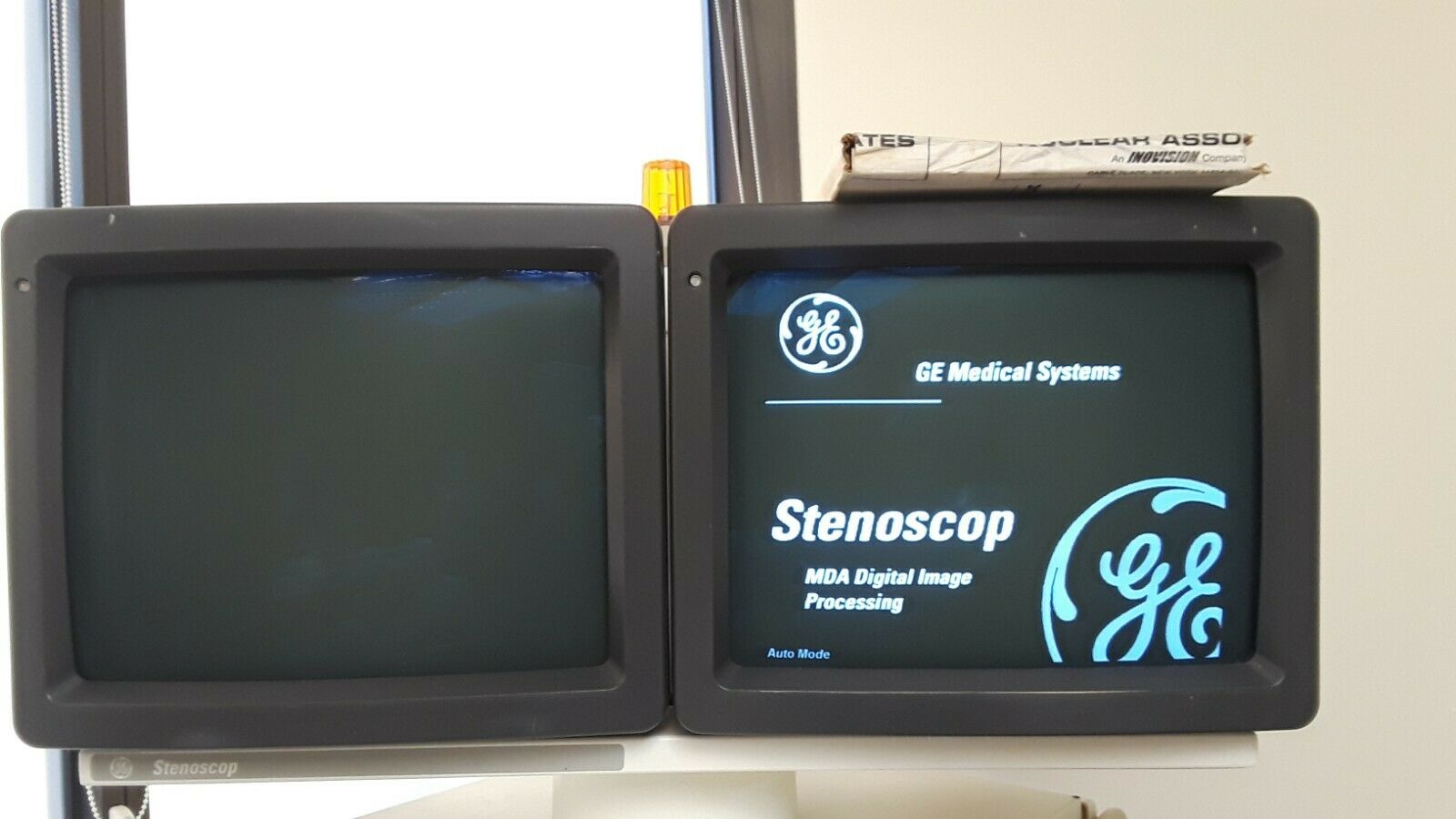
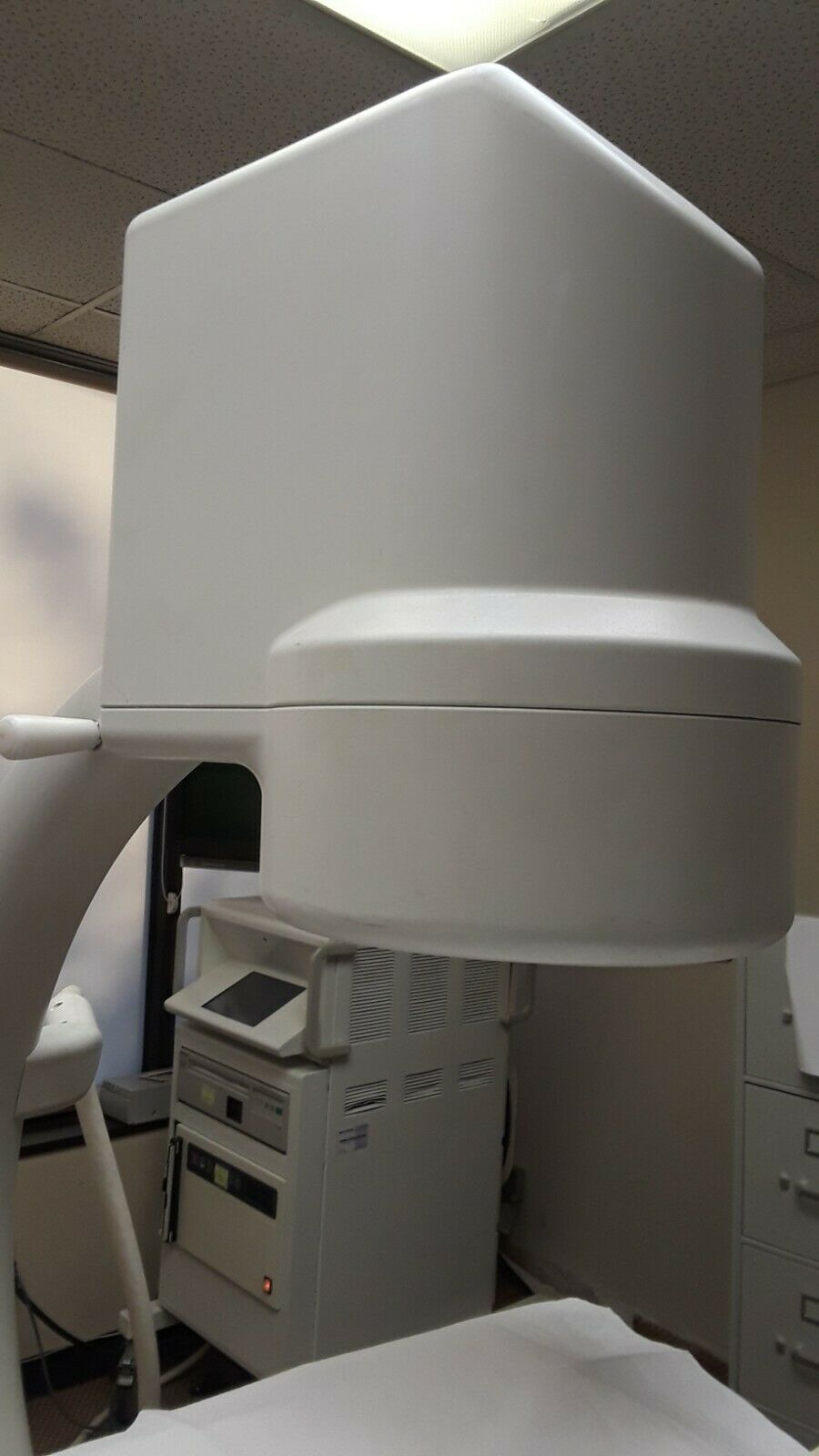
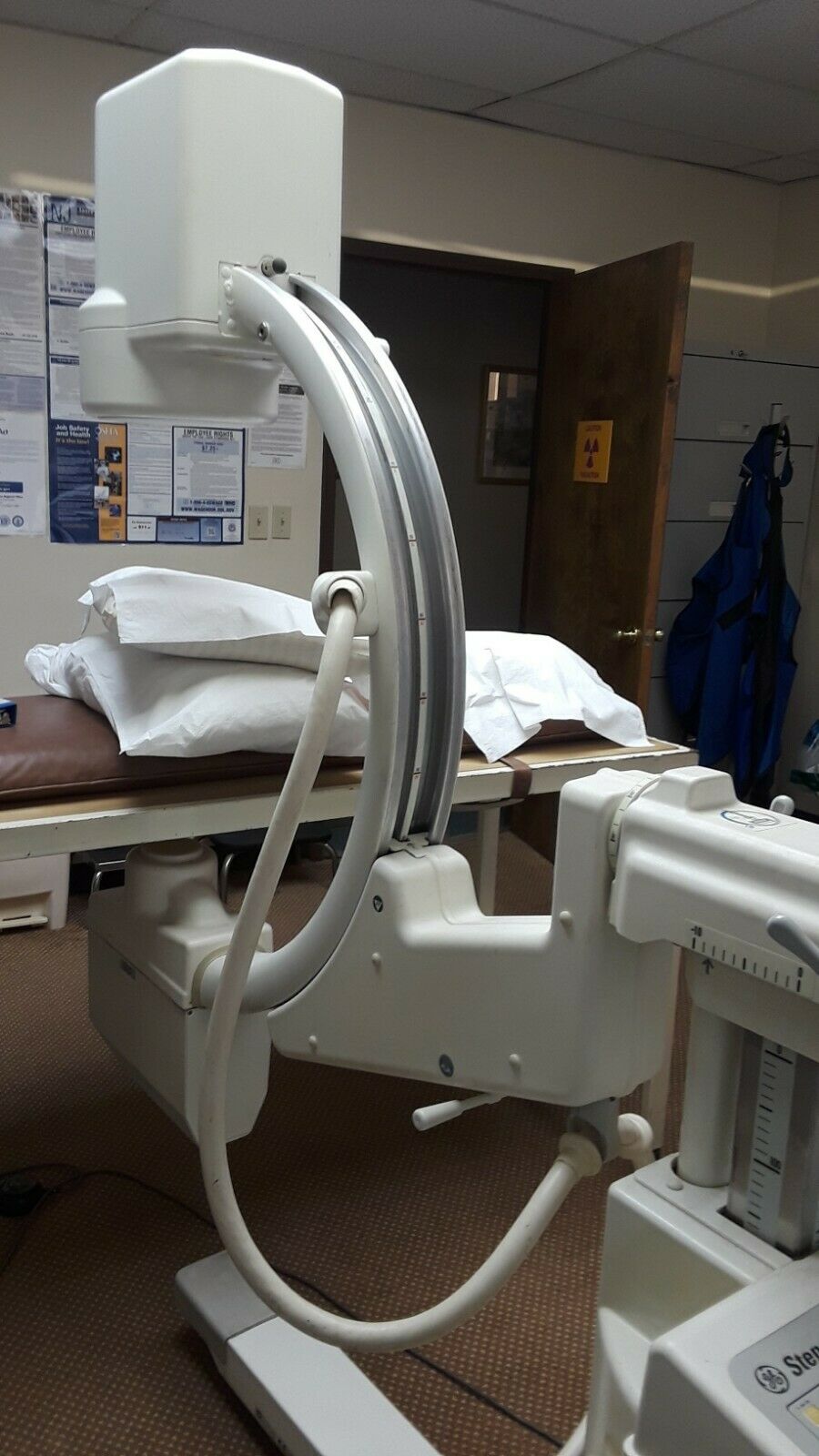
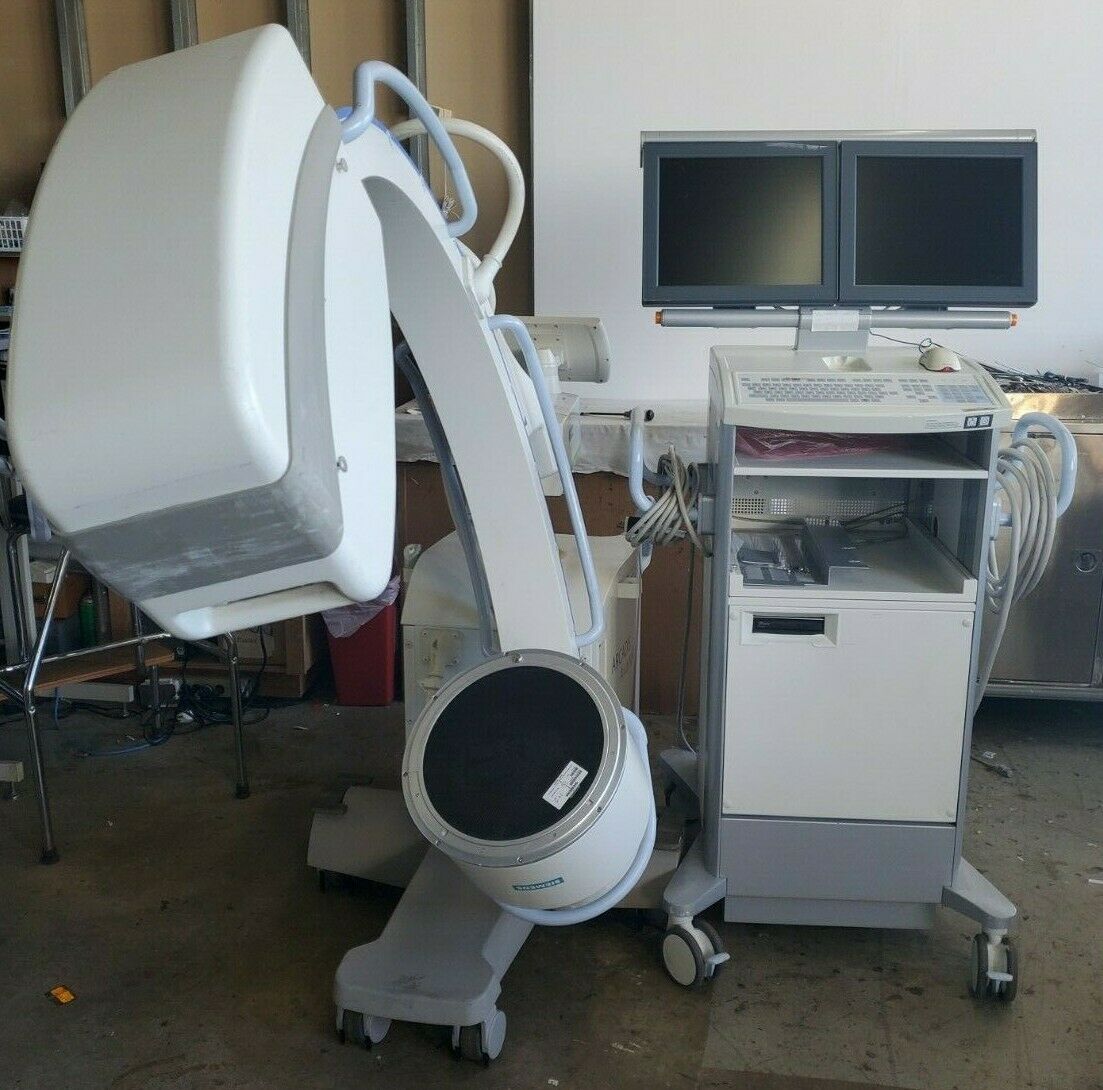
1998 GE Stenoscop MDA Digital Image Processing C-arm in good working condition
$ 4224
- Description
- Size Guide
Description
Up for sell is a 1998 GE Stenoscop C-arm in a closing down pain management office in New Jersey. It is a working system with MDA Digital Image Processing with a 9" Image Intensifier and a 6" magnification zoom. Maximum 110 kVp and 5 mA system. Some cosmetic problem on covers (see pictures) but do not interfere with performance. Comes with a 2019 detailed physicist report (all passed). High Contrast Spatial Resolution for average adult in 9" FOV is 1.8 lp/mm and
in 6" FOV is 2.0 lp/mm. The Low Contrast Resolution
for average adult can resolve in 9" FOV of 1.6 mm diameter hole and
in 6" FOV can resolve a 1.0 mm diameter hole. It comes with a SONY thermal video graphic printer (UP-897MD). It comes with TWO (2) full lead aprons (FREE). If needed, a simple fluoroscopic table can be purchased for additional cost (see picture). FREE local pickup preferred. We will file the NJ DEP x-ray equipment ownership transfer form with your name and address when pick up. The Stenoscop c-arm is sold in as-is condition. There is NO warranty for this equipment. We Have to collect NJS Sales Tax of 6.625%.
Please ask questions and I will answer to the best of my knowledge.
The buyer will not hold the seller, eBay or manufacturer responsible for the appropriate or inappropriate use of this device either by the user or a third party.
"The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item."